collaborating with Epygenix, Polaryx, and Forest Hills Lab.
Pipeline
We will contribute to creating a healthy society.
ENG



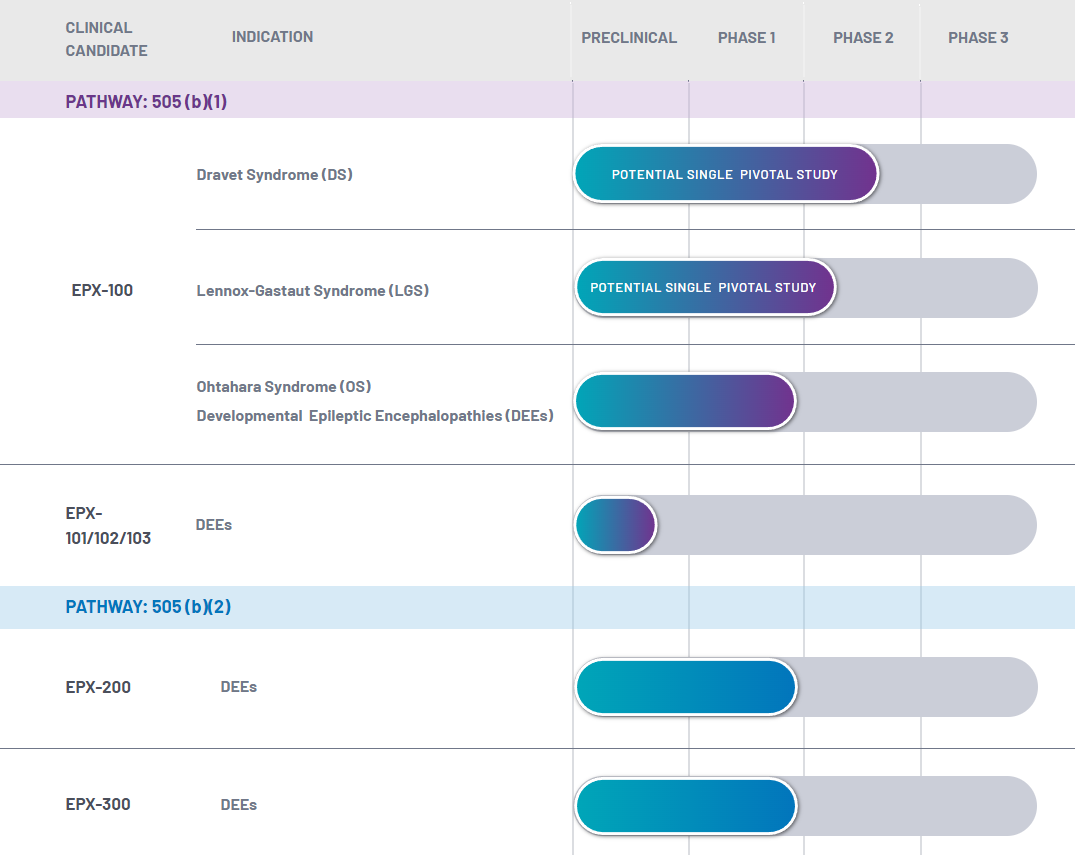

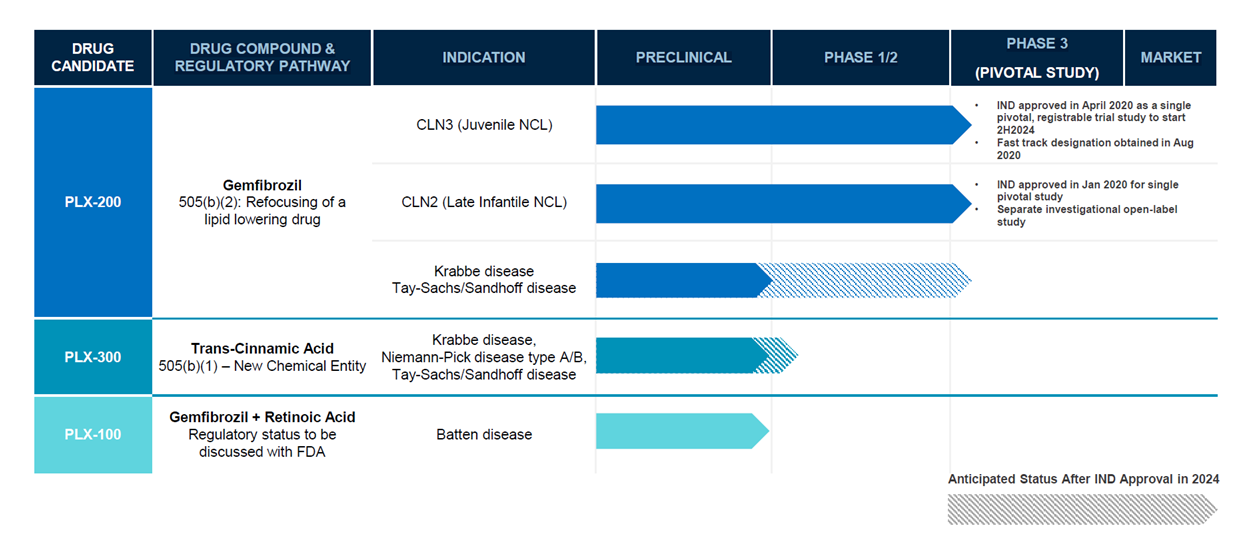
| Project | Indication | Pre-clinical research | Phase 1 clinical trial | Phase 2 clinical trial | Phase 3 clinical trial |
|---|---|---|---|---|---|
| EPX100 | Dravet syndrome |
|
|||
| Lennox-Gastaut syndrome |
|
||||
| EPX200 | Dravet syndrome |
|
|||
| Lennox-Gastaut syndrome |
|
||||
| EPX300 | Dravet syndrome |
|
|||
EPX100 |
2020년 10월 임상 2상 시작. US FDA에서 505(b)-1 (신약허가) 로써 신약 임상 2상 허가 완료
임상 2상에서 총 24명 환자에게 투여 하면 되기에, 회사 현재 schdeule은 2021년 여름~가을경에 initial read-out 결과가 나오며,
2021년 가을~겨울경에 임상 2상에 대한 final report 완성을 목표로 하고 있슴.
LGS질병에 대한 IND filing (신약 임상 허가)도 허가 받았기에, LGS 질병에 대한 임상 2상은 2021년 여름 정도에 시작하려고 준비 중임. 2021년 LGS 질병에 대한 임상 2상 시작 |
|---|---|
EPX200 |
EPX200 로 임상 2/3상 으로 진행예정. Locaserin은 기존 약물이기에 재창출 약물 (US FDA 505(b)-2)로 인정 받았으며, 그리하여 임상 1상 면제 확답 받음.단, locaserin이 2020년 초, 암 발생 위험이 있다는 FDA의 경고에 따라, 현재 검토 중에 있슴. EPX300: 항우울제인 trazodone 으로서, US FDA와의 pre-IND meeting 완료 되었으며, IND filing 허가를 받으면 바로 임상 2/3상 으로 진행예정. Trazodone 또한 기존 약물이기에 재창출 약물 (US FDA 505(b)-2)로 인정 받았으며, 그리하여 임상 1상 면제 확답 받음. |